Several alumni and faculty members have received FDA approval for devices and procedures in recent years — and are preparing to do it again.
(text and background only visible when logged in)
The flight from Atlanta to Los Angeles is full. As luggage feeds into the plane’s belly and passengers take their seats, the captain claims her own. She’s flown the ATL-LAX route countless times and is ready for another. As she sits and looks at the control panel, preflight checklist finished, there’s nothing more to do but start the engines.
That’s because the captain is about to fly cross-country without a flight plan. The 777 is unequipped with communication devices, so there will be no contact with air traffic control. Nor is there radar, which means no warning signs of bad weather. The captain will rely solely on what she sees out her window, guided by landmarks and compass direction.
This, of course, isn’t how pilots fly planes. But it is how doctors implant cardiovascular devices surgically or with catheters. Surgeons go into a procedure without knowing how well the device will perform or if the patient will experience complications, much like an airline captain guided solely by their skill and eyesight.
Unless, that is, a surgeon uses a tool created by a Georgia Tech faculty member to improve health outcomes for patients with heart disease. Lakshmi Dasi has developed software that uses artificial intelligence (AI) to predict what will happen on the operating table — before doctors even touch their patients.
The software is one of many tools, devices, and procedures created in recent years by College of Engineering researchers and alumni that have received clearance from the Food and Drug Administration (FDA) and are making a real difference in people’s lives.
The trek from an idea to the lab to FDA approval is often long and full of twists and turns. But these Georgia Tech community members have done it at least once — and they all plan to try again.


Sticking needles into eyes isn’t as rare as it might seem. And doing so can be good for you.
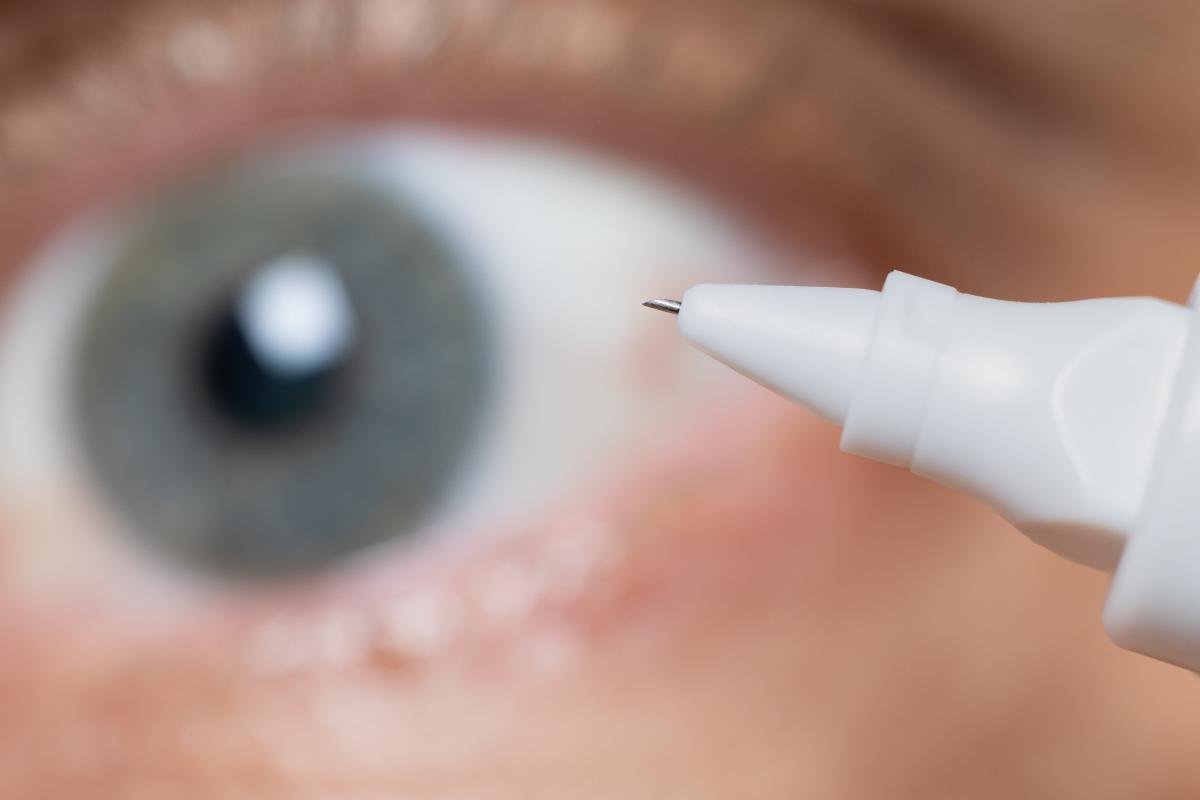
Doctors routinely inject steroids into the eye to treat conditions such as macular degeneration and retinal vein occlusion. The needles are up to 16 millimeters (more than a half-inch) in length.
However, medicine administered into the eye via needles can’t be easily targeted. Injected steroids flood the entire organ, which can cause cataracts and glaucoma.
Regents’ Professor Mark Prausnitz and his research team found a more effective way of injecting medicine almost by accident in 2006. They were using very small needles to insert a depot of drugs into the sclera, the white part of the eye that is firm and difficult to penetrate. Then one day a newer student tried. He found it very easy.
“He knew he did something wrong,” said Prausnitz, the J. Erskine Love Jr. Chair in the School of Chemical and Biomolecular Engineering. “It turns out that the student pressed the needle across the sclera and into the area — the suprachoroidal space — behind it. When a needle is pressed in that space, the injected fluid fills a pocket behind the sclera that keeps the medicine in place and away from other parts of the eye that can cause side effects.”

Prausnitz
The “mistake” led the team to create hollow microneedles that are just a millimeter long. They’re tailored to penetrate the eye only as far as needed to deliver the drugs to targeted internal spaces. Prausnitz and his collaborators at Clearside Biomedical received FDA approval in 2021 and launched the first microneedle product for ocular injections in 2022.
In addition to hollow microneedles for the eye, Prausnitz’s work has led to solid microneedle patches for long-acting contraception, microneedle particles to treat dermatological disease, and microneedle electrodes for mRNA vaccination.
“One of my goals as a Georgia Tech faculty member is training students in the lab and classroom to allow them to launch successful careers. Another goal is doing innovative research that can be published to advance science and engineering,” Prausnitz said. “But I won’t be satisfied if those are my only contributions. I want my research to go beyond Georgia Tech and into the medical community in order to make a difference in healthcare.”
(text and background only visible when logged in)
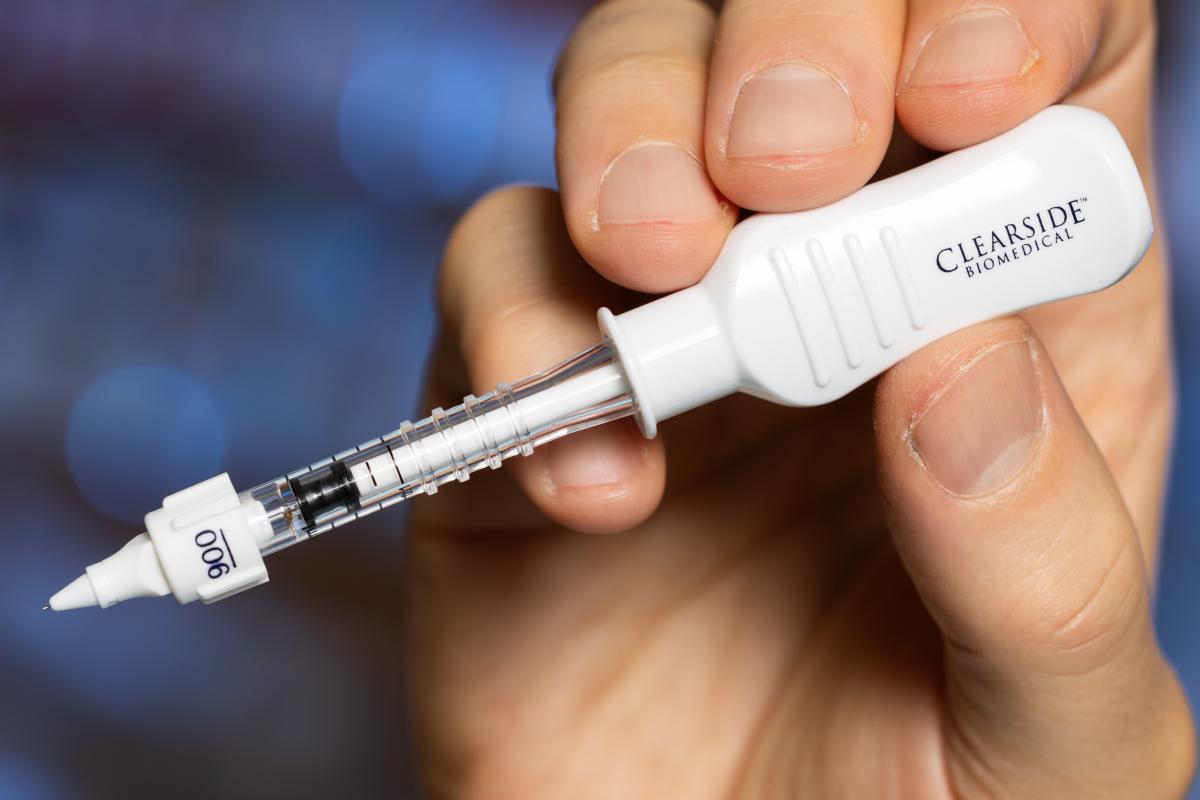
The hollow microneedle is just a millimeter long. (Photos: Candler Hobbs)



Tyburski
Erika Tyburski made a career-defining decision after her senior design project wrapped up in 2012. The biomedical engineering major had created an instant anemia test and was thinking about potential commercialization.
“I had two options: Go into industry for a cushy job, make lots of money, and pay off my student loans,” Tyburski said. “Or I could go on a different path and see where the project, AnemoCheck, would take me.”
Tyburski walked that second route, and it’s worked. She’s now the chief executive officer of Sanguina, a company with a digital platform and physical products that include AnemoCheck. The company has 14 employees spread across the country and is headquartered in Peachtree Corners, just a few miles northeast of Georgia Tech’s campus.
People suffering from anemia lack enough healthy red blood cells or hemoglobin, a protein that carries oxygen from the lungs to the body’s organs. Having anemia can cause fatigue and weakness. The AnemoCheck test measures hemoglobin levels with a single drop of blood. The technology, which finished second at the 2013 Georgia Tech InVenture Prize, was cleared as an in vitro diagnostic by the FDA in 2017 to allow clinicians to diagnose anemia.
Tyburski, who is herself anemic, said she’s a different person than the one who stood on the InVenture Prize stage 10 years ago.
“I was once afraid to ask questions,” she said. “I’ve learned not to be afraid. I ask questions and learn. I have the right team around me, and I know I’m the right person to run this company.”
Sanguina also has created a digital platform and smartphone app for anemia management. The company’s current goal, shaped by the increased availability and acceptance of remote healthcare after the pandemic, is to expand the AnemoCheck test offerings so people can test themselves and monitor anemia at home.
“Telehealth used to be a novelty. It isn’t anymore,” Tyburski said. “For anemia and other chronic diseases, remote management is ideal. Nearly a fourth of the world’s population is anemic, and many live in rural areas or low-resource settings. Sanguina’s mission comes down to access — providing access to our tools. If we can get our technology into the hands of those who need it, people will become more empowered to take greater control of their own health.”
(text and background only visible when logged in)

Georgia Tech’s InVenture Prize competition was one step on the way to commercialization for Erika Tyburski and AnemoCheck. (Photo: Rob Felt)
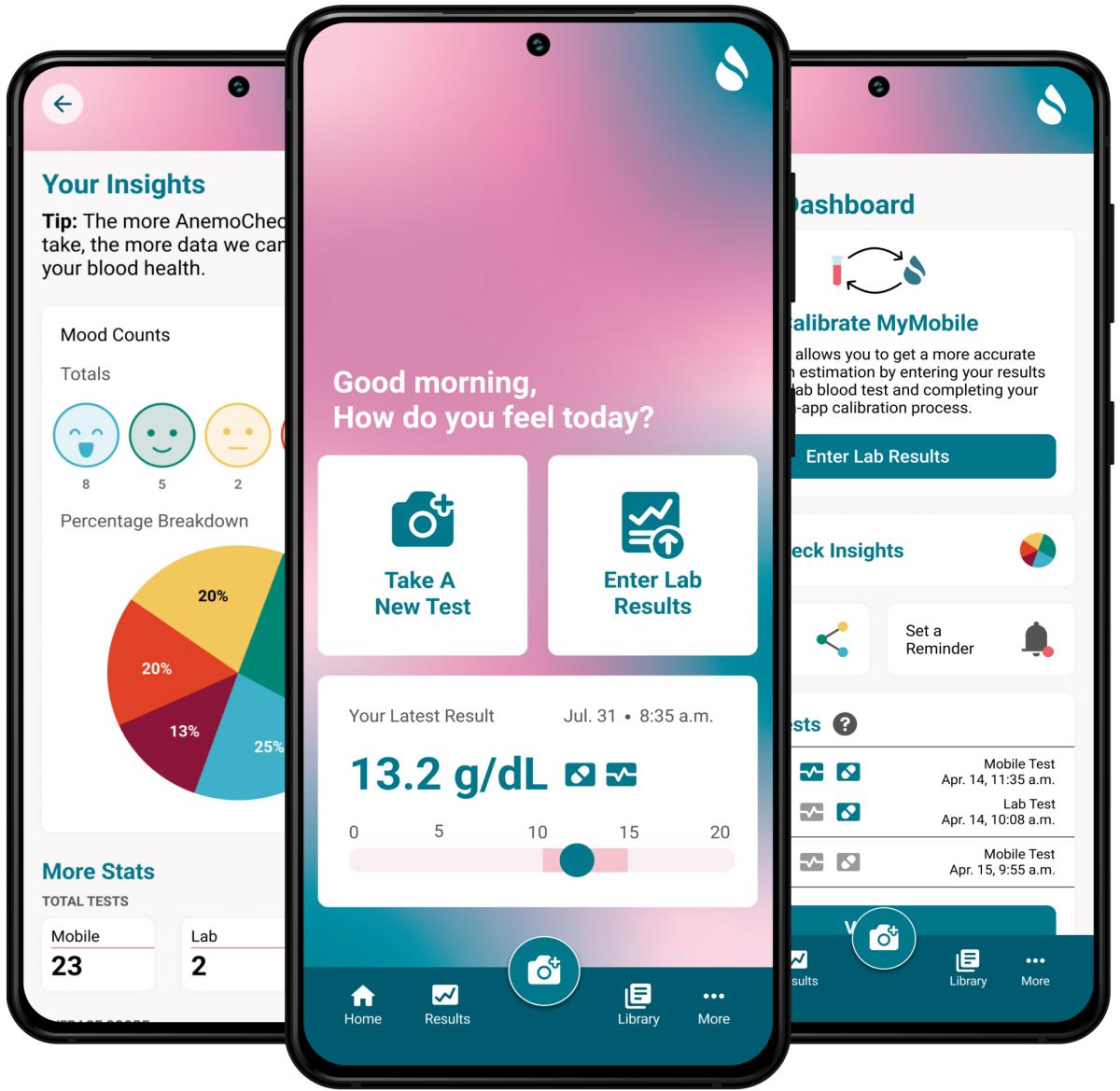
The app makes it easy for users to manage anemia at home. (Image courtesy: Sanguina)


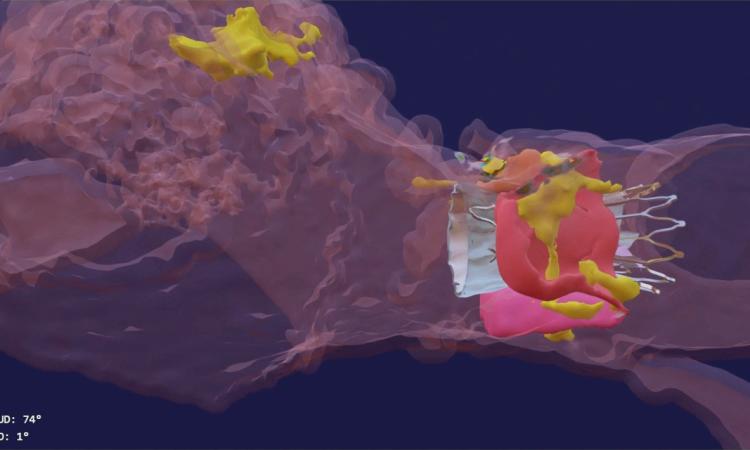
The most recently FDA-cleared technology born from Georgia Tech research comes from Lakshmi “Prasad” Dasi’s lab.
His company, DASI Simulations, received the go-ahead in summer 2023 for its first product, called Precision TAVI. The software tool uses CT scan angiograms to build 3D models and an interactive platform for cardiologists to simulate heart-valve surgery before they even step foot in the operating room.
“The surgeon uses the software to interact with a digital twin of the patient and make predictions about how the surgery might go,” said Dasi, the Rozelle Vanda Wesley Professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “This is the first tool available to physicians to visualize the interaction between various devices and each patient’s unique anatomy before surgery.”
Dasi said nearly every device-related complication in heart valve surgeries is due to an imperfect interaction between the patient’s organs and the implant. Heart tissues can be stressed during some surgeries. In others, abnormal blood flow can prove fatal. With Precision TAVI, doctors can see possible ruptures before they happen.

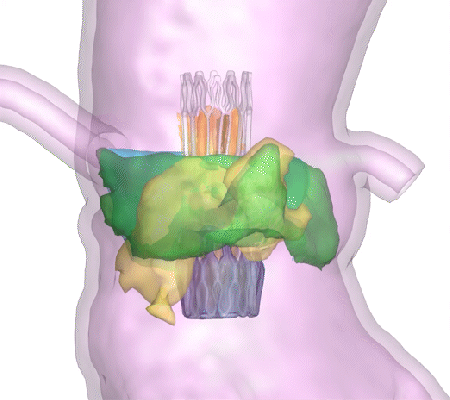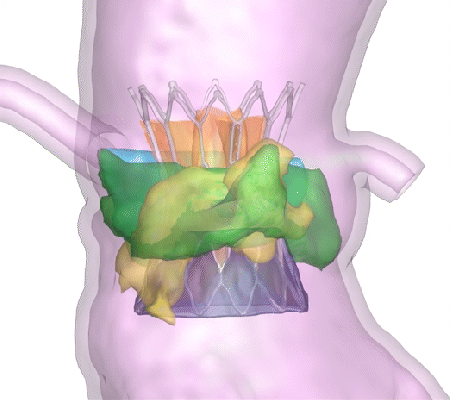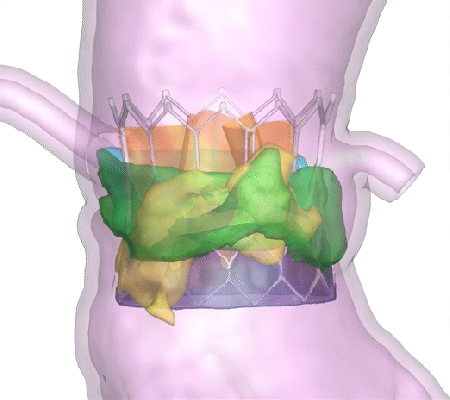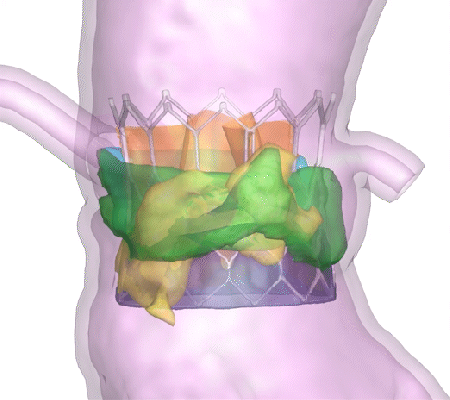
“Hindsight is always 20/20 when it comes to complications during or after heart valve surgery,” Dasi said. “One surgery at a time, our technology helps avoid complications and repeated interventions, helps with lifelong planning for optimal structural heart care, and has the potential to save billions in unnecessary costs from preventable complications.”
The early prototypes of the AI interface were made from commercial software. However, Dasi said such a setup can’t be scaled and personalized. That’s not ideal when doctors replace 100,000 heart valves every year. Dasi and his team developed a second-generation approach that added the ability to personalize the simulations, then tested it with patients at Piedmont Hospital in Atlanta.
“When Georgia Tech makes it possible for engineers like me to collaborate with doctors and have the resources and freedom to pursue high-risk tech, that’s when innovation happens,” Dasi said. “We were blessed to have such a free environment to develop our ideas and bring them to reality.”



Ruff and Kolovich
Greg Kolovich earned his electrical engineering degree in 2004, then went to medical school at Ohio State and Harvard and became a hand surgeon in Boston.
Day after day, he looked at X-rays of traumatic hand injuries. The more he saw, the more he recalled his Georgia Tech experience.
“Once you become an engineer, you never stop being an engineer,” Kolovich said. “You’re always looking to make things better and solve problems.”
As a hand and orthopedic surgeon, he became frustrated about how difficult it was to get X-rays for his patients. They would frequently have to wait several days for an appointment, then drive to his office, often from long distances.
“If technology could shrink a computer into a phone that could be taken anywhere, surely we could miniaturize an X-ray machine to make it more widely available,” Kolovich said.
That’s when he called his former Tech classmate Evan Ruff. Within five years, the duo had started OXOS Medical and created the world’s first handheld, dynamic X-ray system. Ruff said the device, which is in use in 50 facilities nationwide, is much more than a smaller machine. It’s the start of an effort to redefine the future of radiology and make healthcare accessible to more people.
“X-ray machines shouldn’t be limited to hospitals and doctors’ offices,” said Ruff, OXOS’s CEO, who finished his computer engineering bachelor’s in 2003 and earned his Tech MBA four years later. “We want to put them in retirement homes, schools, at sports fields, and in developing nations. Our cloud-based technology can streamline the entire process, with images sent to doctors working remotely, allowing them to see and diagnose within minutes rather than waiting days to see patients.”
The OXOS machine also uses less radiation than typical X-ray machines, the founders said.
“I never lost that engineering curiosity, even while working with X-ray technology that has been around for more than 130 years,” said Kolovich, OXOS’s chief medical officer. “The real value of Georgia Tech was learning about perseverance and not taking things at face value. It’s exciting to help redefine this field and make it safer and more accessible to society.”

The handheld x-ray device may shorten the diagnosis time for patients. (Photo courtesy: OXOS Medical)
(text and background only visible when logged in)
Related Stories
Artificial Intelligence Improves Outcomes of Heart Valve Patients
Prasad Dasi’s 3D modeling and AI simulations help surgeons navigate high-risk surgeries before they even enter the operating room.
Mark Prausnitz Elected to National Academy of Engineering
The honor is one of the highest professional distinctions for engineers.
A Culture of Innovation
Georgia Tech fosters the entrepreneurial ideas of our students and faculty with makerspaces, design competitions, startup incubators, and more.
(text and background only visible when logged in)

Helluva Engineer
This story originally appeared in the Fall 2023 issue of Helluva Engineer magazine.
With the top biomedical engineering program in the country, it’s only fitting that our rebooted College magazine focuses on health and medicine in our return issue. From cancer to anemia, synthetic biology to eye disease, Georgia Tech engineers are improving medicine today and designing a healthier tomorrow for us all.